By Jeremy Yeoh
Illustration by Anne-Gaëlle Goubet for SYIS
One unpleasant aspect of the upcoming summer is the rise of undesirable insects (no offence to entomologists). The advent of the insect season brings upon us much suffering – mainly in the form of itchy and sometimes painful bites, causing pain and inflammation in the form of a red welt. Well, as the saying goes ‘no pain, no gain’ – this itchy bump is a coordinated effort by both the nervous and immune systems actively reacting to danger to protect us (by avoiding and preventing pathogen’s entry). While some people would think of pain as an annoying side effect, the nervous system can play a bigger role in influencing our immunity than one might think and possibly vice versa.
Brief History of NeuroImmunity
Neuron-mediated pain and immune-mediated inflammation have been thought to be linked since around 2000 years ago. Further experiments in the 1800s and 1900s corroborated this hypothesis, where denervation in the skin led to a decrease in inflammation. Given the advances in biochemistry in the 60s, these principles and mechanisms behind this interaction was starting to be understood with the discovery of neuronal mediators of inflammation in the form of signaling molecules. More recently in the early 21st century, the ‘inflammatory reflex’ showed the vagus nerve playing an active role in suppressing the production of a key inflammatory cytokine, tumor necrosis factor (TNF), in splenic macrophages during peripheral inflammation. Interestingly, this phenomenon has been linked also with the elevated inflammatory state in obesity. One area of interest recently for neuroimmunity has been the enteric nervous system (ENS), which surrounds our gut from our stomach to our anus. This so-called ‘second brain’ has two-thirds the number of neurons of a cat, can operate autonomously and can live with or without communication with the parasympathetic and sympathetic nervous systems. As such, the field of neuroimmunity has largely been concentrated on this area.
Deciphering the Code – How two biological systems with different functions speak to each other
As of today, we have observed both seemingly interconnected systems speaking the same language – with immune-associated receptors (PRRs) being expressed on neurons and neuronal-associated receptors (neuropeptides) on a medley of innate and adaptive immune cells. Of recent interest are gut-resident innate immune cells like macrophages, mast cells and innate lymphoid cells (ILCs). During homeostasis, muscularis macrophages (MM) lying close to the enteric nerves can modulate muscular contraction and receive in return sustenance in the form of macrophage colony stimulating factor (M-CSF) by neurons. Other innate immune cells are also involved with neuronal signaling when things start going wrong. During visceral pain, mast cells (most well known for their involvement in allergies) can degranulate upon receiving neuropeptides, where it can drive the sensitization of sensory neurons. These neuronal nociceptors can sense pain through messengers (histamines, prostanoids, inflammatory cytokines) produced by immune cells, indicating a coordinated effort of our body in informing our brain that something is wrong and to take corrective measures.
‘Two’s company, but three’s a crowd’ – Gut Microbiota adding a new facet of regulation to health A major part of the gut that we have not addressed here is our many little friends living with us – our microbiota. It is increasingly clear that our microbiota has widespread effects on our body and to nobody’s surprise, it can also tune and regulate neuroimmunity. Gut immunity is widely reported to be evolutionarily adapted to co-exist with our commensal flora, with neurons in close proximity with the epithelium, microbiota, and immune cells (Fig 1).
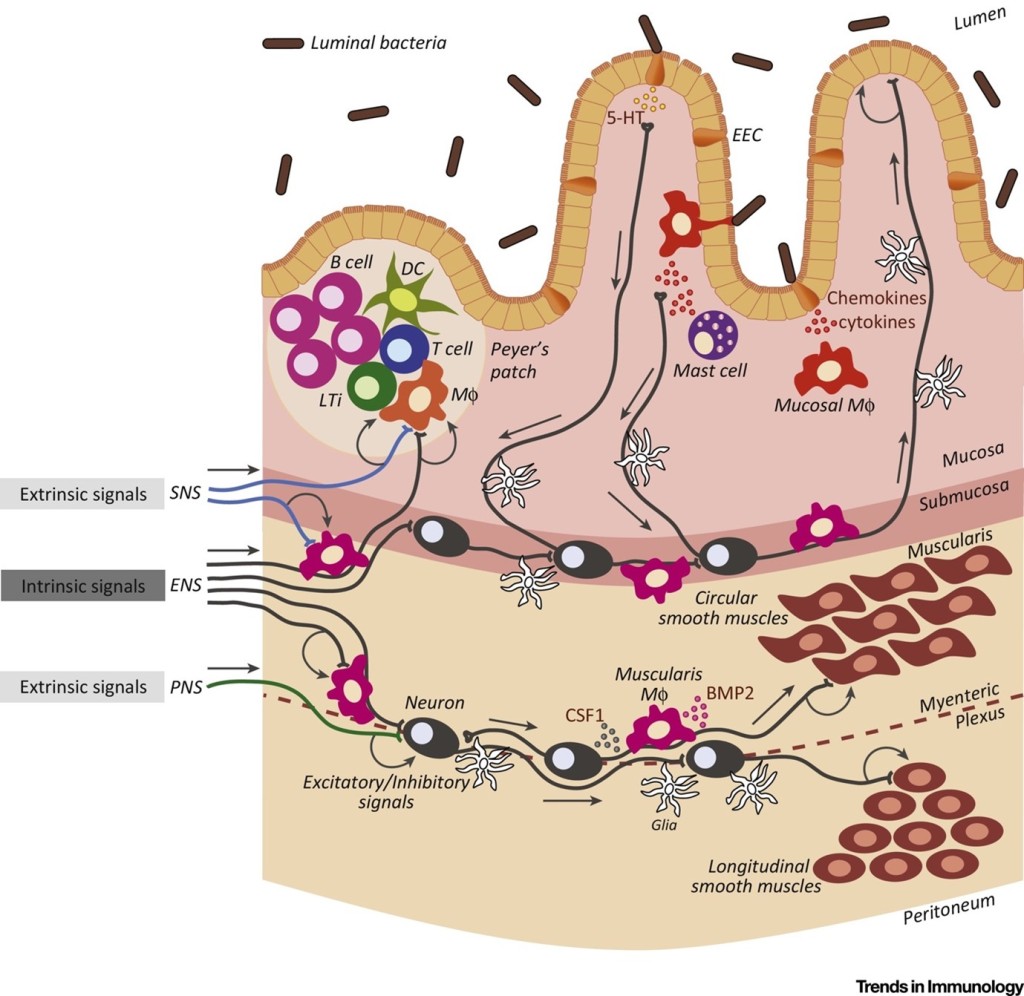
Neurons and immune cells can interact with the microbiota in a variety of ways (PAMPs, metabolites, neurotransmitters). There is also some evidence that suggests that neurons can also regulate the microbiome composition. This triangle of co-regulation between the microbiota, immune cells and neurons is tightly regulated, where any dysregulated compartment will affect the others. Consequently, it makes it a gut-wrenching challenge to determine the origins of certain pathologies in the intestines, where Jacobson et al. further posits that these systems should be interrogated as one integrated system to obtain a better understanding of the overall picture.
NeuroImmunity and Disease
Coming back to pain and immunity, certain diseases have been linked to a dysregulated neuro-immune circuitry, mainly Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD). These diseases come with debilitating visceral hypersensitivity and abdominal pain, severely impacting quality of life. Due to the intense crosstalk between the microbiota, immune system and nervous system mentioned above, it is still unclear which component is responsible for/initiated this dysregulation. Nevertheless, one of the identified major drivers of disease flares in IBS is stress, suggesting that it can be initiated by cues from the brain (via stress hormones).
Exciting Horizons for Treating Inflammatory Diseases
With an increasing understanding of the mechanisms behind neuroimmunity, bioelectric medicine is starting to be implemented for the treatment of certain autoimmune/inflammatory diseases (i.e rheumatoid arthritis). This is mainly done through stimulation of the vagus nerve to manipulate the ‘inflammatory reflex’ mentioned above, via implantation of a small device (pill-sized) into your neck to stimulate the vagus nerve (Fig 2).

While the idea of electrocuting yourself might be a bit shocking, the dose of current released by the implant are minimal as the nerve fibers are extremely sensitive. Currently, this method is still a burgeoning field with a ‘one size fits all’ approach; however researchers/engineers are working on ways to innervate specific organs of interest via targeting specific nerve fibers. Bioelectric medicine has therapeutic potential as a complementary approach to traditional treatments, which often have strong side effects. One of the end goals for bioelectric medicine is the development of a ‘closed loop therapy’, where the device can adapt and decipher the signals between cells and respond appropriately – and this can only be done with more insights on these crosstalks in neuroimmunity. If you find this field of two interconnected disciplines interesting, I highly recommend these reviews (Pain & Inflammation, Crosstalk of Microbiota/Neurons/Immunity, Bioelectric Medicine, Immunology and Psychiatry) that comprehensively illustrate our current understanding in the field.
Main Takeaways
- Complex crosstalk exists between the nervous system and the immune system, that can be tuned by the microbiota in certain organs.
- The study of neurology and immunity as one integrated system in certain diseases may give us a better pathological understanding.
- Future of bioelectric medicine and neuromodulatory drugs may ameliorate inflammatory diseases.
The SYIS does not guarantee the accuracy of the content published in this blog. The content does not necessarily reflect the opinion or views of the SYIS.

Leave a comment